Research Focus Prof. Dominik Schaer:
Hemoglobin Toxicity and Haptoglobin Scavenging
We found that stimulation of endogenous Hp synthesis in dogs prevented Hb-induced hemodynamic responses. Furthermore, guinea pigs administered exogenous Hp displayed decreased Hb-induced hypertension and oxidative toxicity such as to the proximal tubules of the kidney.
The uptake of free Hb by CD163 described here may become relevant once Hp-binding capacity has been depleted by massive hemolysis or on extravasation of erythrocytes.
In a sheep model, administration of haptoglobin into the CSF inhibited hemoglobin-induced cerebral vasospasm and preserved vascular NO signaling. We identified 2 pathways of hemoglobin delocalization from CSF into the brain parenchyma and into the NO-sensitive compartment of small cerebral arteries.
Background
Dominik Schaer studied medicine at the University of Zurich, where he completed his MD thesis at the Institute of Pharmacology.
He specialized in Internal Medicine and Clinical Immunology and currently serves as an attending physician, consulting on systemic inflammatory diseases in the Department of Internal Medicine at the University Hospital of Zurich.
Dominik Schaer is a Full Professor of Internal Medicine and Vice Dean of Education at the University of Zurich.
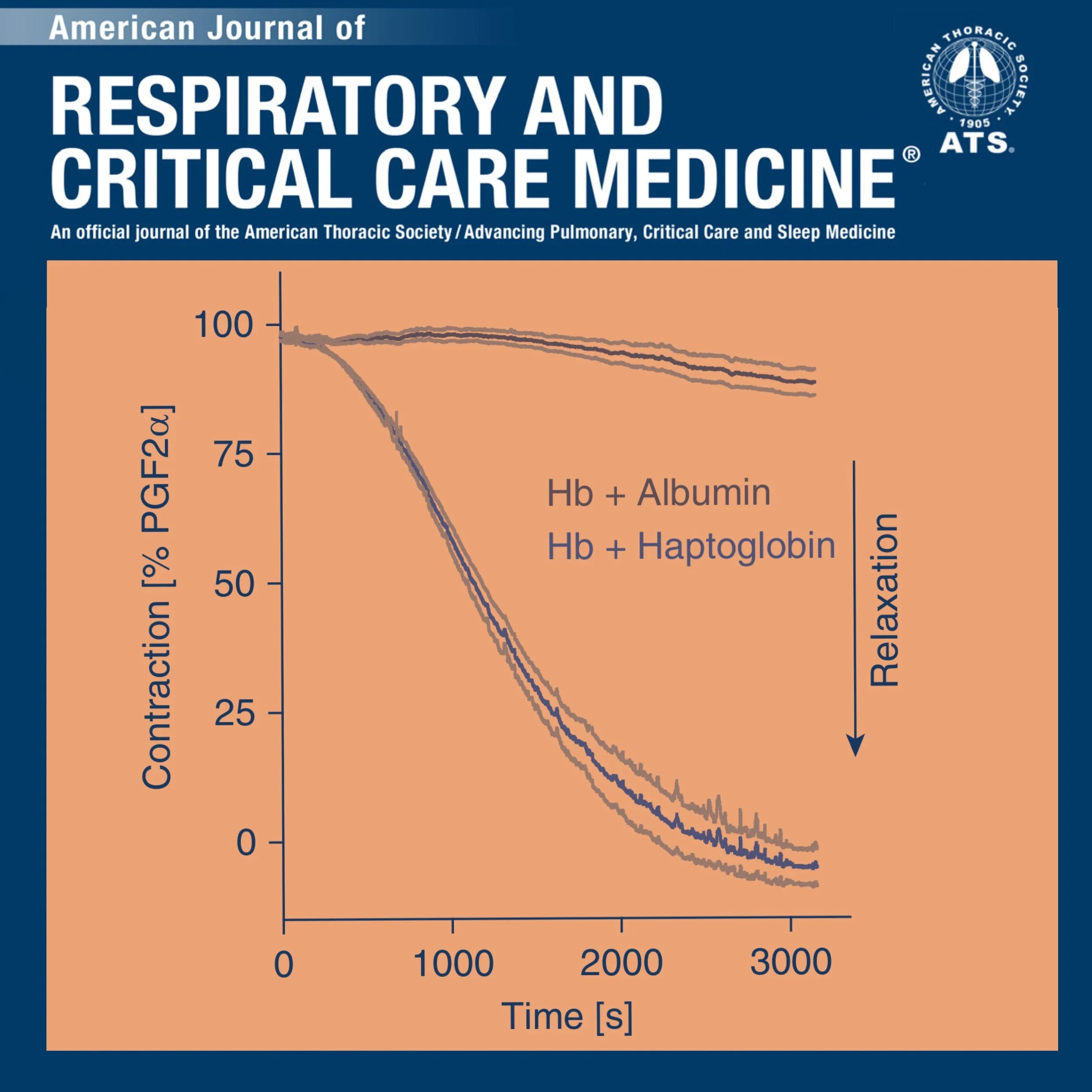
Haptoglobin Preserves Vascular Nitric Oxide Signaling during Hemolysis
The balance of compartmentalization and extravascular translocation of cell-free Hb determines deregulation of vascular nitric oxide signaling during hemolysis.
Supplementation of haptoglobin may support vascular nitric oxide homeostasis and prevent vascular disease complications in patients with hemolysis.
Research Motivation
As a physician-scientist, I’m driven by the challenge of translating cutting-edge science into therapies that improve patient care.
Our translational research program focuses on understanding the complex biology of hemolysis, aiming to develop new treatments that reduce the harmful effects of red blood cell toxins.
As Vice Dean of Education in Medicine, I’m also deeply committed to training and mentoring future physician-scientists. We offer a supportive environment where young doctors can engage in research while advancing their clinical skills.
Research Rationale
I am fascinated by human physiology, particularly how the body manages active substances across different compartments.
My research focuses on red blood cell (RBC) toxins—specifically hemoglobin, heme, and iron—and their role in disease. These toxins contribute to the severity of various conditions, including genetic hemolytic anemias such as sickle cell disease, transfusion-related complications, device-induced hemolysis, atherosclerosis, sepsis, and intracranial hemorrhage.
Haptoglobin
A key achievement in our work has been uncovering how the hemoglobin-binding protein haptoglobin offers protection, paving the way for new therapeutic approaches.
We are now testing these therapies in advanced preclinical models and moving toward clinical trials for conditions like hemolysis-related acute kidney injury during cardiac surgery, sepsis, and aneurysmal subarachnoid hemorrhage (aSAH).
In parallel, we are leading a multinational clinical study on the role of cell-free hemoglobin in causing secondary brain injury in aSAH patients. The goal is to validate it as a biomarker and determine threshold levels that may require treatment.